For Original Drug Manufacturers 丨 How to React to the Early Drug Dispute Resolution Mechanism
On 4 July 2021, the National Drug Administration and the National Intellectual Property Officejointly made an announcement on the (Provisional) Rules on Implementation of the Early DrugPatent Dispute Resolution Mechanism (“Early Dispute Resolution Rules”) followed by thepublication of related rules the Regulations of the Supreme People’s Court on Several IssuesConcerning Applicable Law on Dealing with Civil Cases of Patent Rights in Drugs and theAdministrative Decision Making Rules in the Early Drug Patent Dispute Mechanism. This marksthe completion of the Chinese drug patent linkage system, which will have a significant influenceon both original and generic drug manufacturers. The issues of haste market launch of genericdrugs with their techniques covered up and a lack of motivation to break original drug patentbubbles are to be resolved at an early stage. This article looks at different approaches to theseissues from the angle of original drug manufacturers.
I. Early Dispute Resolution Rules gives ways to overcome the dilemma facing original drugmanufacturers as in the Bolar case
The exception of the Bolar case stated in Article 75.1(5) in which production, use or importationof patented drugs or medical instruments to provide information needed for administrativeapproval or production or importation of patented drugs or medical instruments for that purposeoriginal is not seen as patent right infringement used to cause trouble to drug manufacturers.
For instance, in the NBP Pharmaceutical of CSPC Group v. Limin Medicine Factory of Livzon Groupinvention patent infringement case ((2019) ZGFMS No.2178), the original drug manufacturer NB claimed the courts of first and second trials decided that the generic drug did not infringe thepatent rights simply based on the exception in the Bolar case without ascertaining whether thegeneric drug fell into the protection scope of the patent rights so that the patent owner had noway to protect its rights. However, the supreme court held that the act of Limin applying for thegeneric drug registration to the drug examination center was in essence a request for theadministrative authority to grant an administrative license, not the act of “implementing a patent”defined in Article 11.1 of Patent Law and therefore should not be seen as an infringement of thepatent rights.
In the Sandoz (China) Pharmaceutical Ltd. v. Jiangsu Hanson Pharmaceutical Group Ltd. Inventionpatent right case ((2019) M.01 M.C.No.2796), the generic drug manufacturer Hanson took partin centralized purchasing of medicine before patent rights in the related original drug expired.The court found that it offered to sell the medicine and infringed the patent rights. Withoutevidence of having actually sold the medicine within the valid period of the patent, Hanson wasfinally ordered to pay to Sandoz reasonable expenses of RMB150,000 for stopping theinfringement.
Generic drug manufacturers can apply for registering a generic drug a long time before therelated patent expired. Lured by huge profits, they may hastily launch the drug on the marketafter the grant of approval for it. However, the patent owner has nothing do with this. Thesituation may be ameliorated after the Early Dispute Resolution Rules was taken into action. Thecourt could be asked to give an order to stop a generic drug entering the market before therelated patent expires. In addition, huge rewards for breaking the patent bubbles such as a 12-month exclusive market occupation period will fully motivate generic drug manufacturers tochallenge patents. The competition is ready to begin.
II. What can original drug manufacturers do to win the competition with generic drugmanufacturers?
1. What they need to do basically and about patent information registration
According to Article 2 of the Early Dispute Resolution Rules and categories of registered chemicaldrugs included in the Requirements about Classification and Filings of Registered Chemical Drugs,the Early Dispute Resolution Rules applies on the condition that (1) the original drug is registeredand marketed in China and the generic chemical drug is related to Class 4 registration applications;and (2) patent information related to the original drug is registered with the Chinese MarketedDrug Patent Information Registration Platform (https://zldj.cde.org.cn/home “Platform”).
Therefore, the first step of introducing the original drug into the Chinese market should be closelyfollowed by the second step of registering the patent information on the Platform as soon aspossible.
Besides chemical drugs, holders of market launch licenses for traditional Chinese medicine andbiological products can have related patent information registered. A total of 787 original drugsaltogether, including 524 (67%) chemical drugs have had patent information registered with thePlatform by 1 September 2021, as shown by the graph 1 below.
Types of drugs registered in patent information
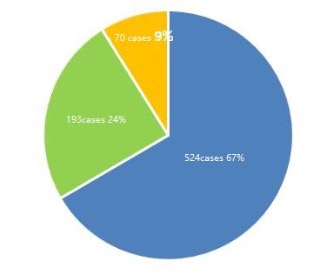
Blue: chemical drugs; Green: traditional Chinese medicines; Yellow: biological products
Graph 1
Holders of market launch licenses for chemical drugs, for example, need to register details of theholder, drug and patent (including patents on the compound used as the active ingredient, the drug combination containing the active ingredient and medical uses and the relationship betweenthe drug and the related patent claims). The graphs 2-5 below is Boehringer registered patentinformation of the drug “afatinib dimaleate tablet”.
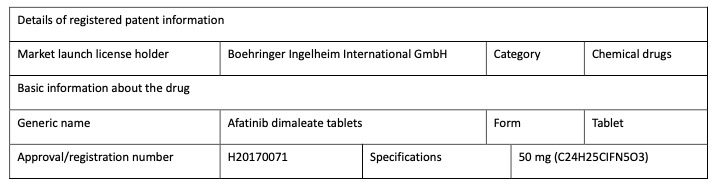
Graph 2
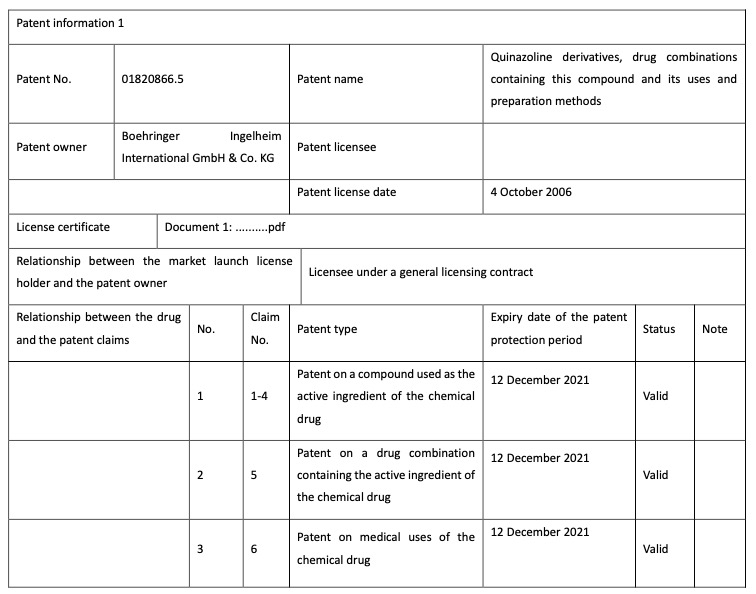
Graph 3
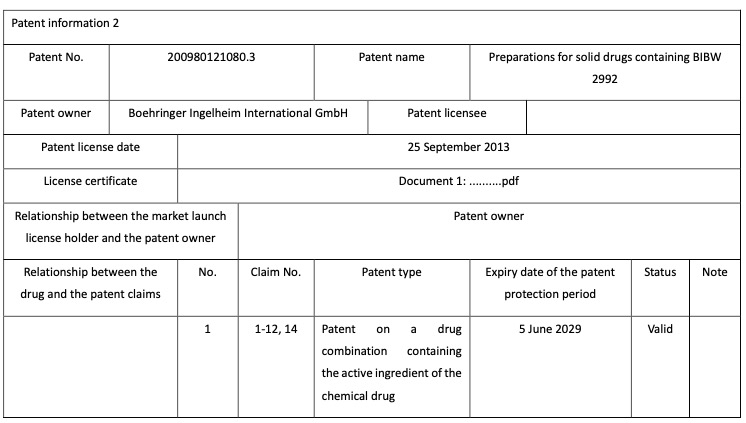
Graph 4
Graph 5
Technical and legal departments need to work together to choose from related patents andclaims to register on the Platform. Professional advice of external lawyers may also beindispensable.
2. What could original drug manufacturers do in the face of a “challenge” from generic drugmanufacturers?
(1) Competition between original drug manufacturers and generic drug manufacturers
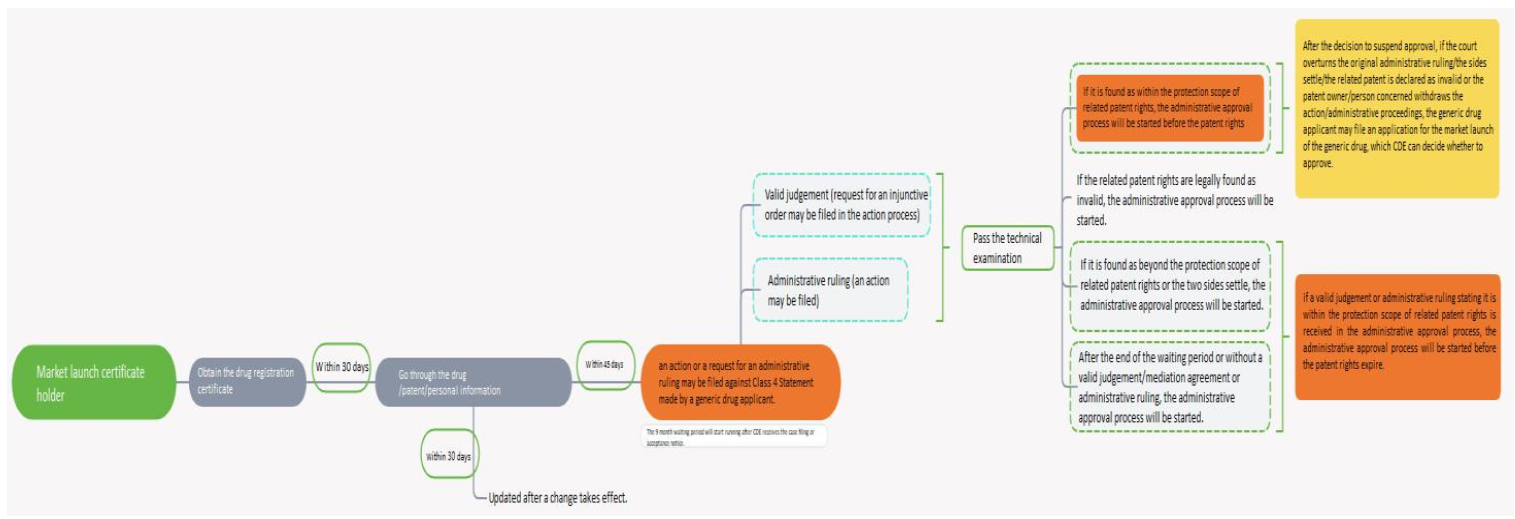
When a generic drug manufacturer makes Class 4 Statement by claiming that “patent rightsrelating to the copied drug should be declared as invalid or the generic drug does not fall withinthe protection scope of the related patent rights”, the battle begins in the map below.
Graph 6
For the layout, the above chart only describes the process that applies in cases where an originaldrug manufacturer (market launch license holder files an action or administrative proceedingsagainst Class 4 Statement made by a generic drug applicant). If the generic drug manufacturersubmits Class 1, 2 or 3 Statement or the original drug manufacturer does not file an action orarbitration against Class 4 Statement, CDE could immediately decide whether to grant approvalfor market launch based on the technical assessment result and the category of the patentstatement without respect to the 9-month waiting period.
Based on Graph 6, for generic drug manufacturers, except the event described by Article 9.2 (1)of the Early Dispute Resolution Rules that for a generic chemical drug registration application thatis found to fall within the protection scope of related patent rights, the administrative approvalprocess could not be started until the patent rights expire (so that approval for market launch isimpossible to obtain in advance), in the other events, legal benefits may be equal to or betterthan those in the Bolar exception, especially considering the 12-month exclusive marketoccupation period.
For original drug manufacturers, the situation is completely different. The worst result is thattheir patent rights are declared as invalid and no longer protectable and a large number of genericdrugs appear. The generic drugs that do not fall within the protection scope of the patent rightscould be approved and marketed. The best result is that the administrative approval process forthe generic drugs could not be started until the patent rights expire. If the generic drug manufacturers finishes the quality control evaluation and take other actions in advance andcompletes the administrative approval process as quickly as possible, the effect of this on themis almost the same as that of patent cliff which existed before the Early Dispute Resolution Ruleswas taken into action.
(2) Generic chemical drugs have the 12-month exclusive market occupation period on thecondition that 1 first successful patent challenge+2 initial grant of approval for market launch.If a patent challenge is successful, the generic drug manufacturer will be required to submit Class4 Statement, the patent rights will be declared as invalid based on its request for the invalidity ofthe patent and approval for market launch of the generic drug will be granted.
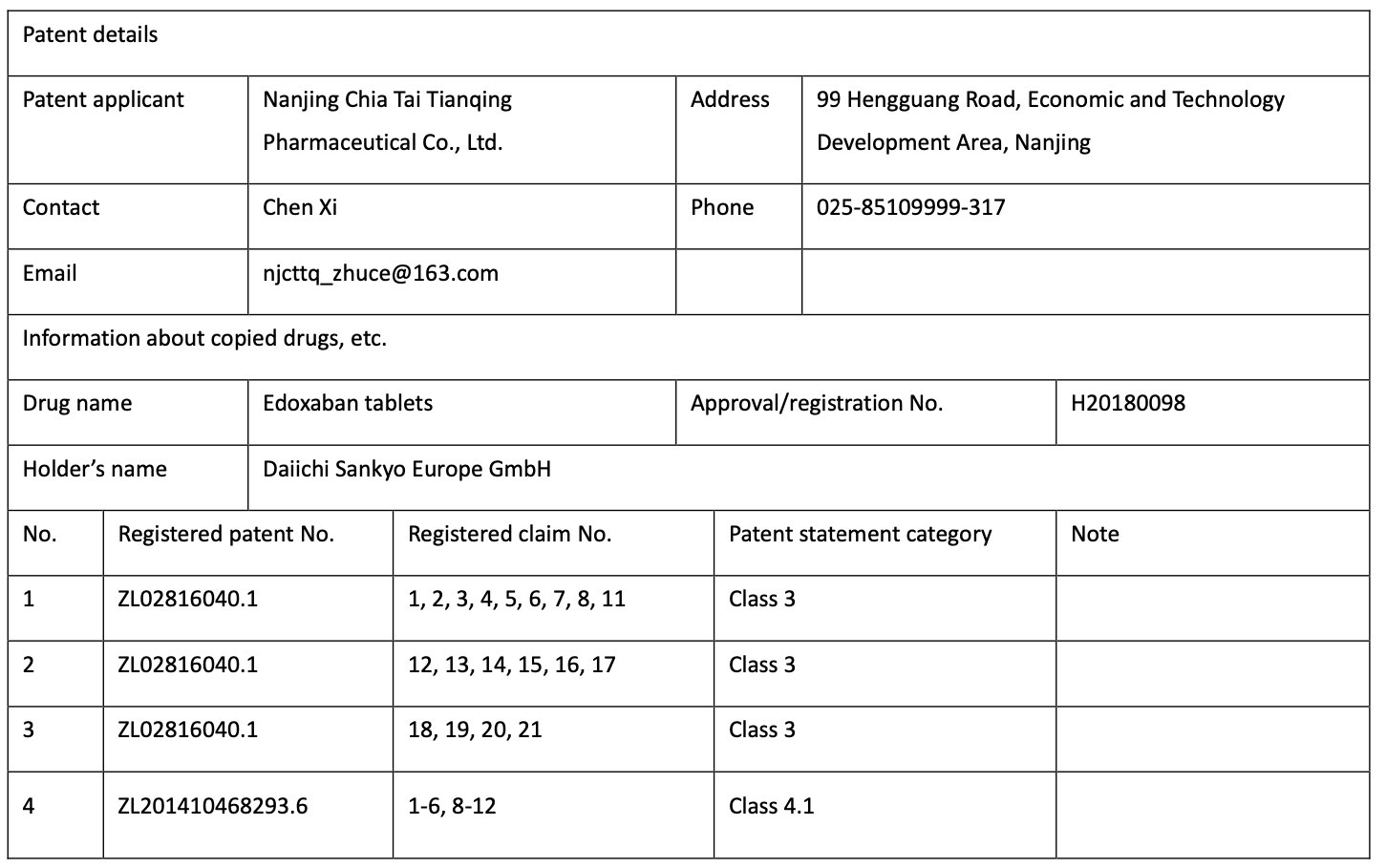
I found no generic drugs registered with the Platform may have the exclusive market occupationperiod based on the above conditions. The best example is “edoxaban tablets”, a generic drugproduced by Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd. on the basis of the original drugof Daiichi Sankyo Europe GmbH, a European subsidiary of Daiichi Sankyo Company Limited inClass “3+4.1” Patent Statement as Graph 7 below.
Graph 7
With the patent rights under Class 3 Statement, even if all the patent rights under Class 4.1Statement are declared as invalid, Chiatai Tianqing would not meet all the conditions on“successful challenge”. Other generic drugs (such as latuda (lurasidone HCL) tablets of ZhejiangHuahai Pharmaceutical Co., Ltd. and “farxiga tablets” Jiangsu Hanson Pharmaceutical Group Co.,Ltd.) which only have Class 4.2 Statement or Class 3+4.2 Statement without going through theinvalid patent declaration process are even further away from meeting the conditions of theexclusive market occupation period.
Therefore, original drug manufacturers need to consider which category the patent statement ofa generic drug manufacturer falls into before deciding what to do.
(3) Impact of the centralized volume-based purchasing system on original drug manufacturers
Article 2 (7) of the Instructions of the General Office of the State Council on Further Implementingthe Centralized Volume-based Drug Purchasing Routine and System (GBF [2021] No.2) states that“regarding generic drugs having passed the quality control evaluation, original drugs andreference drugs, there are no quality groups, generic names used as competitive units in thecentralized volume-based purchasing and no protection or discrimination clauses”. Article 5 (14)states that “original drugs, reference drugs and generic drugs having passed the quality controlevaluation with the same generic name should comply with the same medical insurance paymentrules”.
On 29 January 2021, Chen Jinpu, deputy director of the National Healthcare Agency toldjournalists at the regular briefing meeting on the state council’s policies that if the number ofmanufacturers of an original drug and copies of the drug is up to three, the drug may be put onthe centralized purchasing list”. The criteria for the centralized purchasing are “1 (originaldrug)+2 (generic drug)”.
An original drug manufacturer may make friends with the first one of generic drug manufacturers(that is most likely to be given the exclusive market occupation period), but must treat others asrivals. Otherwise, they would face the centralized purchasing and be asked for a much lower pricesoon.
In this sense, in the face of a dilemma in a patent case, it may be advisable for the original drugmanufacturer to let the generic drug manufacturer a 12-month exclusive market occupationperiod to have a longer time before being put into the centralized purchasing basket.
3. Patents or technical secrets not registered may also be useful
Besides three kinds of patents (i.e. patents on active compounds, medicine combinationscontaining active ingredients and medical uses) that should be registered under the Early DisputeResolution Rules, chemical drugs also include patents on preparation methods, preparations,intermediates and crystals. Original drug manufacturers can bring infringement actions againstapproved generic drugs on the market that infringe any of the above patents.
In addition, technical secrets may make original drugs better and are often helpful for their highpopularity after patents expire. Businesses should work hard to maintain technical secrets in dailyoperations.
III. Other Factors Important to Original Drug Manufacturers
1. Extend valid periods of patents
Article 42 of Patent Law states that an additional period of patent rights may be given at thepatent owner’s request as a compensation for unreasonable delay of the invention patentapproval or new drug evaluation and approval process.
Therefore, original drug manufacturers should not ignore this important part of the patentapplication and administrative approval processes. They should regularly check expiration datesof patents so that they can file requests for additional periods of time in a good time.
2. Be aware of data protection and other related laws.
Article 34.2 of Regulations of Implementing Drug Law states that drugs containing new chemicalingredients will have a six-year data protection period after approval for its market launch. ThePolicy on Encouraging Creation of New Medicines and Medical Instruments and ProtectingCreators’ Rights (Exposure Draft) states more specifically that besides creative medicines,medicines for orphans and children are to be given a period of time for data protection. TheRegulations, once taken into action, will benefit original drug manufacturers no less thanstrengthening protection of their patents.
Therefore, we suggest original drug manufacturers keep informed of latest legal information.
IV. Conclusion
In my opinion, the Early Dispute Resolution Rules encourages original drug manufacturers tolaunch original drugs on the Chinese market in the shortest possible time to diversify drugsavailable in China and gives original and generic drug manufacturers a good mechanism to resolvepatent disputes at an early stage. During the competition with generic drug manufacturers,original drug manufacturers should consider the following factors when deciding what to do.
1. Make sure your patents are not found as invalid. Valid patents are the basis for all things. Checkexpiration dates of patents regularly from an early stage.
2. Give technical and legal support. If technology used in a generic drug does not fall within theprotection scope of the related patent, the administrative approval process for the drug will belinked with the expiration of the patent term so that the patent cliff may appear at a later time.
3. Settle with the first generic drug manufacturer in an appropriate way or even let it have theexclusive market occupation period;
4. Give accents on maintaining technical secrets and protecting data of original drugs.

 沪公网安备 31010602001694号
沪公网安备 31010602001694号